
The proven leader in the SCIg market1
Connect with us
Meet with your CSL Behring Associate Director of Corporate Accounts
to explore the value of our leading subcutaneous
immunoglobulin (SCIg) therapy.
Meet with your CSL Behring Associate Director of Corporate Accounts to explore the value of our leading subcutaneous immunoglobulin (SCIg) therapy.
Schedule a meetingFormulary kit
Review product-specific resources for Hizentra.

Explore the value of Hizentra
Learn more about CSL Behring’s immunoglobulin (Ig) products for the treatment of PI and CIDP.
Read nowWhat is Hizentra?
Hizentra is indicated for:
- Treatment of primary immunodeficiency (PI) in adults and pediatric patients 2 years and older.
-
Maintenance therapy in adults with chronic inflammatory demyelinating polyneuropathy (CIDP)
to prevent relapse of neuromuscular disability and impairment.
– Limitation of Use: Maintenance therapy in CIDP has been systematically studied for 6 months and for a further 12 months in a follow-up study. Continued maintenance beyond these periods should be individualized based on patient response and need for continued therapy.
For subcutaneous infusion only.
What are the key benefits?
#1 PRESCRIBED
#1 prescribed Ig for PI and the only 20% SCIg approved for CIDP2
MARKET SHARE
Hizentra is the market share leader in new starts and switches* and holds more than ~58% of the SCIg market share1†
PROVEN EXPERIENCE
Proven experience since 2010 and more than 14.9 million exposures worldwide1‡
COMPARABLE WAC
The wholesale acquisition cost (WAC) of Hizentra is comparable to that of other SCIg products3
*Market share data January 2020 through December 2023.
†Based on volume share of SCIg brands through December 2023.
‡Estimate based on Hizentra grams sold worldwide, across all indications, from 2010 through March 2024.
Overview of disease states

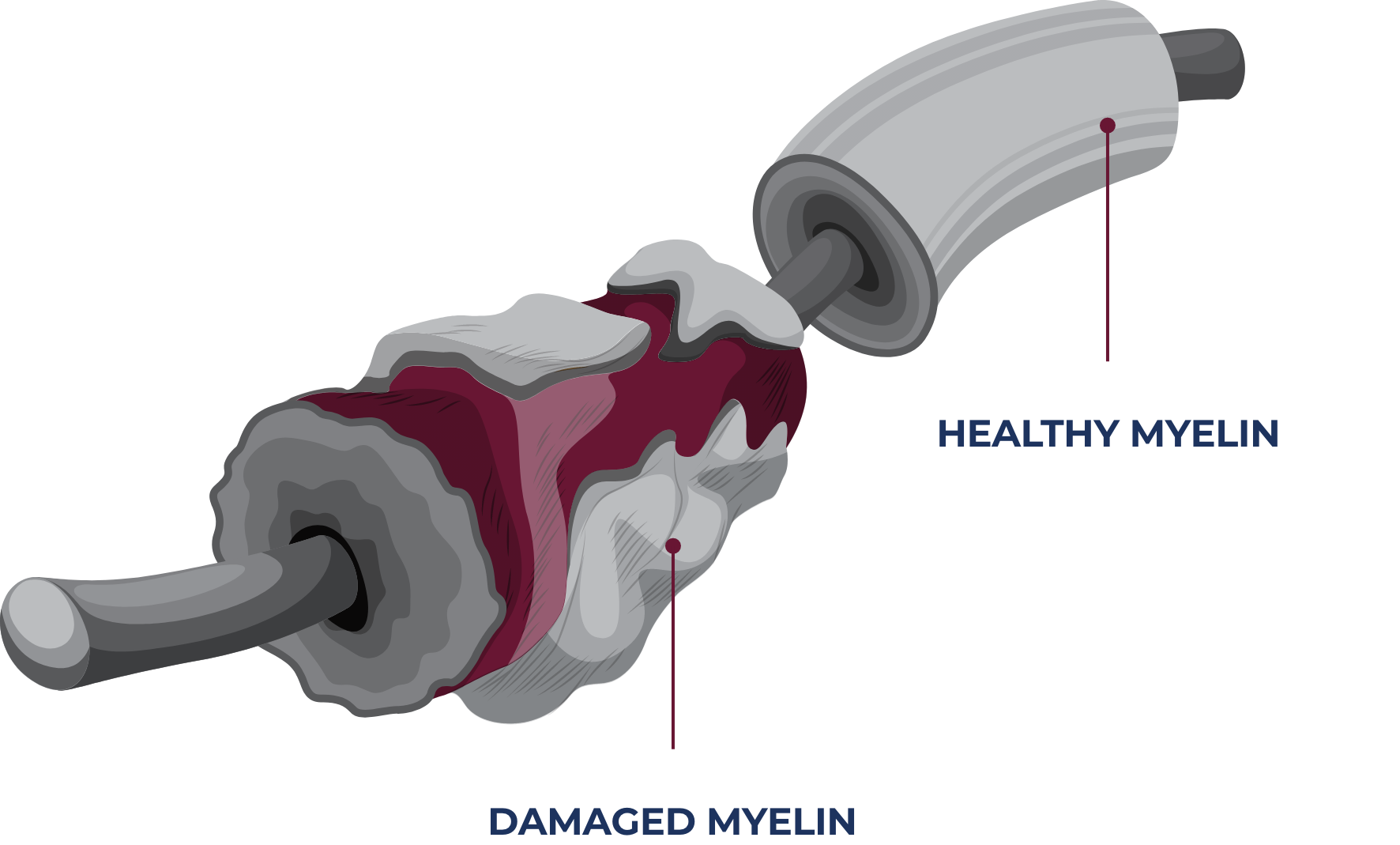
About CIDP
CIDP is a rare, progressive, and disabling autoimmune disease of the peripheral nervous system, with an estimated prevalence of 1 to 9 per 100,000 individuals.4-6 The disease attacks and destroys myelin, a material that protects the axons that transmit electrical signals throughout the body.7,8 This causes signaling impairment, leading to a loss of strength and sensation in the arms and legs.4,5,7,8
CIDP affects the peripheral nervous system—
all nerves that connect the spinal cord and brain with other tissues and organs in the body.9
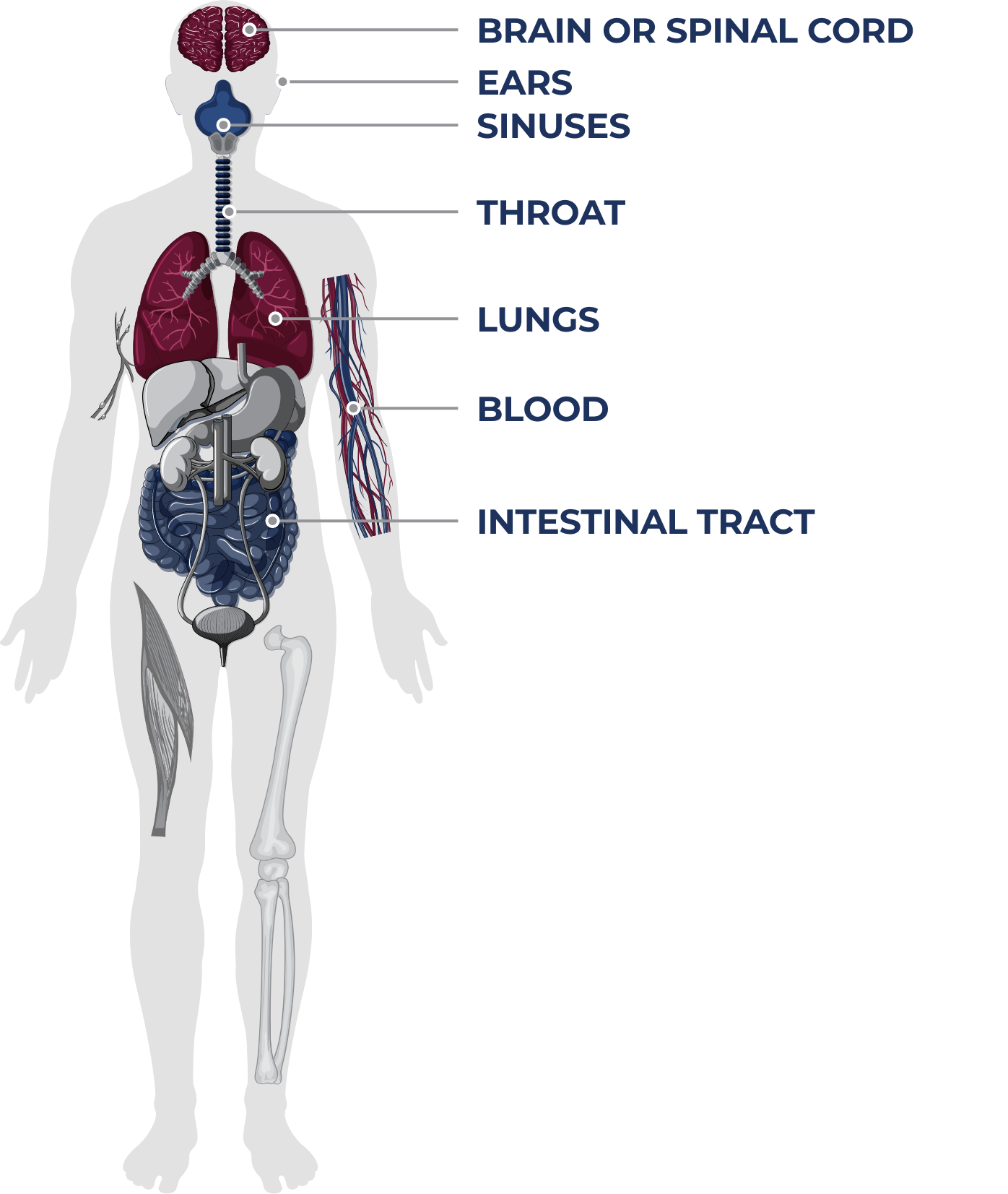
About PI
PI includes numerous rare, chronic disorders that increase the risk of persistent infections. There are more then 485 different forms of primary immune deficiency diseases affecting approximately 500,000 people in the United States.10, 11
PI is caused by a variety of genetic defects in which part of the body’s immune system is missing or impaired.12 Untreated patients are at increased risk of severe, persistent, and recurrent infection,12,13 which may occur at various locations throughout the body.
It has been shown that by simply diagnosing and treating PI, healthcare utilization costs can be reduced, saving an average of more than $50,000 annually.14
Some patients may benefit from a site-of-care shift
Shifting the site of care from a hospital setting to a home-based setting can also reduce healthcare costs.15
Intravenous immunoglobulin (IVIg) home infusion may significantly reduce site-of-care–related administration costs and improve adherence.16-18
SCIg may be a valuable alternative for patients who19:
- Have venous access issues
- Experience IVIg-related adverse events
- Want more flexibility or find IVIg inconvenient
The question then becomes which therapies are clinically sound and convenient enough for administration in a home-based setting. When choosing IVIg vs SCIg for home administration, considerations should also include systemic side effects the patient has experienced, infusion schedule, and venous access issues.20 CSL has developed Ig therapy options with an eye toward improving the patient experience and providing value and savings to the healthcare system.
Want to learn more?
Meet with your CSL Behring Associate Director of Corporate Accounts.
Help us help you
Share your thoughts so we can further tailor our content.


